Abstract
Context. AML is an oligoclonal disease. Intratumoral genetic heterogeneity (ITH) is pervasive in cancers and has often been linked to poor outcome. Except in patients (pts) with complex karyotypes (Bochtler, J Clin Oncol 2013), the clinical relevance of ITH remains unknown in AML. We assessed ITH in a real-life AML cohort.
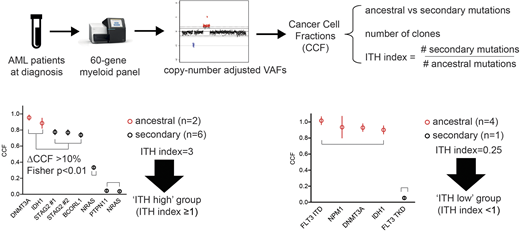
Methods. Since Nov 2015, all newly diagnosed AML pts at our institution were analyzed by targeted sequencing with a 60-gene myeloid panel at an average depth of 815X. Variant Allele Frequencies (VAF) were adjusted for copy number variation to estimate Cancer Cell Fractions (CCFs) and rank mutations by decreasing CCFs. Mutations were segregated into different clones when distant by >10% CCF. Ancestral (as opposed to secondary) mutations are defined as those belonging to the first clone (Figure). We could next derive for each pt the total number of clones and the ITH index, defined as the ratio of secondary to ancestral mutations (according to Turajlic, Cell 2018).
Results. We studied 201 consecutive AML pts (median age 67 years, secondary AML 24%). The median number of mutations was 4 (range 0-12). Clonal heterogeneity (>1 clone) was found in 71% of pts with a median of 2 clones per pt (range 1-5). 140 pts received intensive chemotherapy (IC), 50 hypomethylating agents (HMA) and 11 best supportive care (BSC) only.
Signaling (FLT3, NRAS, KRAS, KIT, JAK2, CBL, CSF3R, BRAF, NF1, PTPN11, RIT1, CALR and MPL, 60%), DNA methylation (DNTM3A, TET2, IDH1/2, 54%), NPM1 (29%), spliceosome (U2AF1, SRSF2, SF3B1, ZRSR2, PRPF8, 27.9%) and tumor suppressors (PHF6, TP53, WT1, 27.4%) were the most frequently mutated pathways. ELN 2017 risk category was favorable, intermediate, adverse and not evaluable in 35%, 27%, 35% and 3% of pts respectively. Increasing ITH was associated with a higher number of clones (p=0.001) and of mutations (p<0.001). ITH was greater in pts with at least one mutation in histone modifiers (ASXL1, ASXL2, EZH2, KMT2A-PTD KMT2D, KDM6A, p=0.0004), cohesin (STAG2, RAD21, SMC3, SMC1A, CTCF, p=0.0018) or signaling (p=0.002) genes but was not affected by the presence of mutations in DNA methylation or spliceosome, tumor suppressor genes, transcription factors (RUNX1, CEBPA, ETV6, GATA2) or co-activators (BCOR, BCORL1, EP300, CREBBP), all p>0.05. ITH was not different among ELN 2017 risk categories (p=0.15). ITH was independent of age, WBC count and type of AML (secondary vs de novo), all p>0.2.
There was no significant difference in the number of mutations (p=0.12), clones (p=0.58) or ITH indexes (p=0.38) according to IC/HMA/BSC treatment strategies. We next performed a preliminary analysis of the prognostic impact of ITH in the 140 pts treated with IC (median age 58y), with a median follow-up of only 12.5 months. 117 (84%) pts achieved CR and 32 (23%) were transplanted in first CR. Median event-free survival (EFS) was 18.7 vs 8.0 months in pts with adv and non-adv ELN risk, respectively (p=0.016). In univariate analyses, DNA methylation (p=0.04) and spliceosome (p=0.007) mutations were the two pathways predicting adverse outcome. The number of mutations (p=0.6) or of clones (p=0.8) had no impact on EFS. Pts with fewer secondary than ancestral mutations (ITH <1, 'ITH low' group, n=64 [46%]) had a median EFS of 13.6 months vs 19.8 months in the 76 (54%) pts with ITH index ≥1 ('ITH high' group, p=0.067). In a multivariate Cox model accounting for age, log(WBC), ELN risk, AML type (secondary vs de novo), presence of DNA methylation and spliceosome mutations and ITH, ITH-high was identified as a significant predictor of improved EFS (HR=0.54, p=0.03), independently of older age (HR=1.02, p=0.07), adv ELN risk (HR=2.4, p=0.005) and higher WBC count (HR=1.27, p=0.004). Censoring at transplant in first CR did not affect this result. In 14 pts who relapsed after a median of 12.9 months since diagnosis with paired NGS at relapse, the number of clones significantly decreased at relapse (p=0.046).
Conclusion. Our results confirm that most AMLs are oligoclonal. Surprisingly, higher ITH is not related to older age or secondary AML. Our preliminary results suggest that lower ITH translates into shorter EFS in pts treated with IC. Longer follow-up and/or increased pt numbers are warranted to determine whether NGS-based assessments of clonal heterogeneity can improve AML risk stratification.
Fenaux:Otsuka: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal